Atoms come and atoms go
Within a nucleus is a complex interplay of forces, some trying to crush the nucleus, others trying to blow it apart. For those few cases when the balance is just right, the nucleus will be stable. Hydrogen-1, oxygen-16, carbon-12 are examples of this. For the vast majority of other occasions, when there is a proton too many, or a neutron too few, the nucleus will be unstable and so will eventually decay. The nucleus is described as radioactive.
THE NATURE OF DECAY
Nuclear decay, otherwise known as radioactivity, is the process of an unstable nucleus transforming into another by ejecting a high energy particle. These high energy particles, generically referred to as nuclear radiation, are capable of stripping electrons from their atoms, a process called ionisation and so are a type of ionising radiation along with some radiation from non-nuclear sources such as x-rays and high energy ultraviolet rays.
The concept of radioactivity was first discovered in 1896 by the French physicist Henri Becquerel, who was experimenting with phosphorescence. He identified a previously unknown type of radiation being emitted from uranium salts, which could fog a photographic plate. It came down to Rutherford to identify there were different types of radiation and to start assigning them names. He coined the imaginative terms, alpha ray and beta ray to describe the two main types of radiation emitted from uranium and thorium. The French chemist Paul Villard later discovered gamma rays in 1900 and in 1932, the Italian physicist Enrico Fermi discovered a form of decay involving an unstable atom ejecting a lone neutron. In the years that followed, yet more types were added to the books: proton emission, electron capture, cluster decay, spontaneous fission.
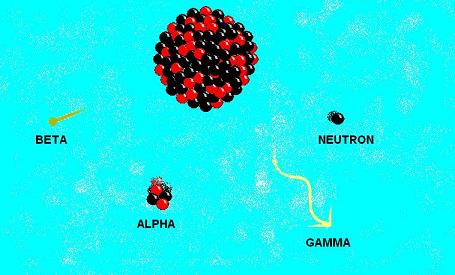
Figure 1- The different types of radiation an unstable nucleus may emit. Alpha, beta and gamma are the common types, but neutron emission can happen certain situations.
Nevertheless, the original three, alpha, beta and gamma, are the most common and the most important, with neutron emission coming a short way behind.
Alpha decay involves the ejection of an alpha particle, which may be more meaningfully described as a helium-4 nucleus, with two protons and two neutrons. An example of this is the decay of radon-222 to polonium-218. The original beta decay, identified by Rutherford, is the emission of an electron from the nucleus as a neutron changes into a proton. Carbon -14 decays by beta emission to become nitrogen-14. Later on, as similar type of decay was discovered involving the emission of a positron, which is essentially a positively charged electron, as a proton changes into a neutron. Oxygen-15 decaying to nitrogen-15 is an example of this. This type is today referred to as beta-positive decay, while the former is often called beta-negative decay.
Gamma radiation is just high energy electromagnetic radiation. It is emitted by a nucleus in an excited state as a way of returning to a lower energy level. The emission of a gamma ray does not itself change the nuclide, but it is often accompanied by alpha or beta decay as well.
Neutron radiation is rarer than the aforementioned forms. This happens when a nucleus is too neutron-rich and needs to spit out a neutron. The products of nuclear fission are often neutron-rich as their parents, being very large, tend to have more neutrons per proton than the daughter elements would commonly have.
THE MEANING OF HALF-LIFE
Nuclear decay is an entirely random process. Looking at any given radionuclide, it is impossible to know exactly when it will decay. It could decay in 5 seconds or it could last for another 5 ages of the universe. However, depending on how unstable it is, we can know how likely it is to decay within a certain space of time. This means that if we have a very large sample, say on the order of trillions of nuclei, we can know pretty accurately how many we expect to decay within that time, or in other words, the rate of decay.
Formerly, the rate of nuclear decay is called activity and it is measured in becquerels, named after Henri Becquerel. An activity of one becquerel means one decay per second. Another unit is the curie, named for the French chemists Pierre and Marie Curie, who did important early work on radioactivity. One curie is defined as the activity of 1g of radium-226, which is around 37 billion becquerels.
Figure 2- Graph showing the size and the activity of a sample of oxygen-15 nuclei. The shapes of the two curves are the same. They both halve at regular intervals.
Since decay is based on probability, the larger the sample we have, the higher the activity we would expect. In fact, the activity is directly proportional to the size of the sample. If we double the sample size, we double the activity. This also means that as a sample decays, its activity decreases proportionally. This behaviour means that for any isotope, the sample, and the activity, will halve at regular intervals. This interval is called the half-life. For example, oxygen-15 has a half-life of 2½ minutes, so after 2½ minutes, you have half of what you started with and after 5 minutes, you have a quarter of what you started with.
Half-life is a reflection of the stability of the isotope. A more stable isotope is less likely to decay quickly and so will have a longer half-life. A more unstable isotope is more likely to decay sooner rather than later and so the half-life is shorter. The corollary of this is that long half-life means low activity and vice versa.
This has important implications for handling radioactive material. If the isotope is very radioactive, it will of course be dangerous if not shielded properly. However, it will also more quickly disappear. For example, in the case of oxygen-15, the high radioactivity makes it a short term hazard, but after just half an hour, there is less than 2.5% of the original sample left. By contrast, an isotope with a long half-life such as uranium-238, with a half-life of 4.5 billion years, will hang around for ages of the universe, but it will also spend that time being only very weakly radioactive and so not much of a hazard.
THE BENEFITS OF RADIOACTIVITY
Once we got past the initial stages of radioactive quackery in which radium and other toxic substances were marketed as medicinal, we successfully implemented radioactivity into a huge variety of processes: medicinal, industrial and domestic. An entire branch of medicine depends on radioactivity, including the use of radioactive tracers injected into the blood for the purposes of diagnostic procedures. Many industries use radioactive material for measurement purposes, from studying the properties of a rock at the bottom of an oil well, to measuring the thickness of paper in a paper mill. Radioactive material even finds an important use in home in the form of smoke alarms, which use the alpha radiation from americium to indicate when the air is clear of smoke (hence when the alarm should not sound).
Radioactive decay can also find a use in the direct generation of heat and power by harnessing it radioisotopic heater units and radioisotopic thermoelectric generators. These have found particular use in deep space missions, for providing heat and electricity at distances from the Sun, where solar cells are insufficient. They commonly use fuel pellets of plutonium-238, which decays purely by alpha emission with a half-life of 88 years. The heat generated can either be used directly to keep the systems of the spacecraft warm as in the case of RHUs or can be use to generate an electric current due to the Seebeck effect as in the case of RTGs.
Ultimately though, this technique is limited. RTGs cannot practically provide more than a couple of hundred watts of power. For generating ener