Splitting atoms
When we talk about “nuclear” reactors, we are generally referring to nuclear fission reactors; reactors, which gain their energy from nuclear fission. Nuclear fission is the process of a nucleus splitting into two or more daughter nuclei. Only the largest nuclei, the ones formed in the collapse of the most massive stars, can undergo fission and release energy in the process. Some really heavy nuclei will spontaneously fission, but that is of little use to us because, for one, a reactor using spontaneously fissioning fuel cannot be turned off, and two, because they fission without prompting, they have long since done so and so are not found in nature. For commercial fission power, we need nuclei that will fission only when prompted to do so. Therefore, we make use of certain isotopes of heavy metals called actinides, which only fission under the right circumstances when they are hit by a neutron.
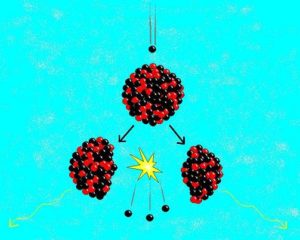
Figure 1- A fissile nucleus is hit by a neutron and fissions to produce two fission products, some neutrons and a lot of energy.
And so our reactors are always made to run on fissile material. The term “fissile” describes a nucleus that will undergo fission when hit by a suitable neutron. In the nuclear reactor, a fissile nucleus is struck by a lone neutron of the right energy, which is then absorbed. The new heavy nucleus then fissions into two (very occasionally three) daughter nuclei called fission products. It will also release two or three more high energy neutrons. Plus of course, there will be a large energy release in the form of movement of the fission products and gamma radiation, which will be absorbed in the reactor as heat in the coolant.
So, we started with a fissile nucleus and a neutron. We now have a bunch of high energy neutrons, a pair of fission products, and a lot of heat. The heat is carried away in the coolant to inevitably be used to turn the generators or else is dissipated elsewhere. The fission products are at an extremely high energy level and are not very happy with their composition and so they are very unstable and hence very radioactive. They rapidly decay by beta and gamma emission and sometimes they even spit out raw neutrons. Because these fission products are producing heat when the fission reaction is shut down, spent fuel must be placed in cooling pools immediately after removal from the reactor to carry away this decay heat. The handy thing though is that because they have such a high activity, they decay very quickly and so after a short time, they are much easier to handle.
Then of course there are the neutrons that were released.
THE REAL MEANING OF GOING CRITICAL
Describing a reactor as critical conjures up scary images of big explosions. In fact, in the context of nuclear power, the term “critical” is a good thing. When neutron strikes a nucleus to cause fission, it is lost from the system. In order to keep the reactor working at a steady power level, the fission must then return a neutron to the system to keep the balance. But an average fission does not return one neutron, it will return between two and three.
This is fortunate because there are some losses. After the fission of a nucleus the neutrons fly off into the other parts of the reactor. Some try to escape and are absorbed in the biological shield. Others get absorbed in control rods, non-fissile fuel or other parts of the reactor. These are lost to the system. Some then go on to strike more fissile nuclei and cause them to fission. The idea is that from the two or three neutrons released from a fissioning nucleus, one of them will do just this. If that is the case, then the balance is preserved.
Say we start with 8,000 neutrons. They all strike fissile nuclei and cause fission. From these 8,000 fissions, we get 20,000 new neutrons. If 12,000 are lost elsewhere and the remaining 8,000 cause more fissions, then the rate of reaction is the same and the reactor maintains its power level. This is criticality. If less than 8,000 neutrons go on to cause more fissions, then the reaction will slow down. The reactor is subcritical and is powering down. If more than 8,000 neutrons go on to cause more fissions, then the reaction will speed up. The reactor is supercritical and is powering up.
THE MANY ENERGIES OF THE NEUTRON
Like all particles, neutrons can be found with many energy levels. When released from a fissioning nucleus, they are at a very high energy level and are called fast neutrons as a result. Fast neutrons are not the easiest things with which to work. Making a reactor, designed to operate only with them, critical is difficult because they are not as effective at causing fissions as slower neutrons. For example, uranium-238 can only be made to fission with fast neutrons, not with slower neutrons (referred to as thermal neutrons). But uranium-235 can be made to fission with thermal neutrons and will do so far more easily than uranium-238 with fast neutrons.
This among other reasons is why the vast majority of current nuclear reactors are so-called thermal reactors, made to burn fissile fuel using thermal neutrons. The problem of course is that neutrons released straight from a fissioning nucleus are fast so in order to make them thermal, they must be slowed down. This is done using a material called a moderator. A moderator is reluctant to absorb neutrons, but will bounce them about quite readily. As they are bounced about, they lose energy and eventually become thermal and can go on to cause fission in the fuel. The most common moderator is light water (plain old regular water to most people), but also used are graphite and heavy water (water with deuterium).
THE YELLOW STUFF
The vast majority of fission reactors across the world exclusively use uranium. Uranium is not an especially rare metal. It is found across the world in one form or another but the highest grade ores are in Canada and Australia, and so those countries are currently at the centre of the uranium market. Because of the energy density of uranium, uranium mining is orders of magnitude less extensive than the mining of coal. The natural uranium recovered from the ground is 0.7% -235 while the rest is -238.
As mentioned before, uranium-235 is easier to make fission because it responds well to thermal neutrons, which are easy to use. Uranium-238 can only be made to fission by fast neutrons and even then, not as easily as uranium-235 with thermal ones. But uranium-238 can absorb thermal neutrons. Upon absorbing the neutron, it becomes uranium-239, which is very unstable and rapidly decays to neptunium-239 and then to plutonium-239, which is also good fissile stuff to use in thermal reactors. That is why uranium-238 is described as fertile. In fact, plutonium does contribute to the energy output of a reactor by being bred and burned in situ, despite not being included in the fresh fuel. If it sits in the reactor long enough without fissioning, it will absorb more neutrons and become plutonium-240 and plutonium-241, which are also useful as fuel in reactors in one way or another, although useless in weapons. Any plutonium not burned in the reactor can, along with any excess uranium-235, be extracted from the spent fuel and be incorporated into fresh fuel or MOX. Ultimately, of course, a thermal uranium and plutonium reactors will always consume more fuel than is bred.
But back to uranium-235. Because thermal reactors spend most of their time burning this particular isotope, its abundance in the fresh fuel is very important. It is possible to make a reactor work with natural uranium (0.7% -235), but it is requires either graphite or heavy water moderator. A light water moderated natural uranium reactor cannot be made critical. Since most reactors around the world are light water moderated, it obviously begs the question of how they get around this problem. The answer is that the uranium is enriched to usually around 3-4% (called low-enriched uranium LEU) using either gas diffusion or the more modern and energy efficient method of centrifuge enrichment. This involves separating out some uranium-238 and removing it from the fuel so that the uranium left has a lower proportion of -238 in it and therefore a higher proportion of -235. This of course produces “waste” uranium-238, known as depleted uranium, which has a variety of uses, some more controversial than others.
But outside of uranium and its reactor descendent plutonium is another raw material that can be used in reactors: thorium. Thorium is three times as abundant as uranium and all of it is the -232 isotope. Thorium-232 is not fissile itself, but it can be bred, much the same way as uranium-238 absorbing a neutron to become plutonium-239, to become uranium-233. Even better, the conversion of thorium-232 to uranium-233 can be done in thermal reactors with a net fuel gain so that more fissile material is in the spent fuel than was in the fresh fuel. Because of its abundance, thorium is seen as the future of nuclear power.
SPEEDING IT UP
Thermal reactors are technically the easiest ones to do, but if we want to make proper use of uranium, we need to make better use of that uranium-238, which is only 1% consumed in the form of plutonium in a regular thermal reactor. In order to do this, we need fast reactors. Despite the technical hurdles, there is a lot of focus on developing economically competitive fast reactor technology. Many fast reactors have been operated commercially around the world, but the lower costs of operating regular light water reactors as well as the low price of uranium means that fast reactors are not as attractive yet.
But fast reactors have advantages. Generally, they are used with plutonium as the main fuel. But they can be used with non-fissile isotopes to burn them and breed them into more fissile material. In this way, they can make total use of uranium and the actinides produced during reactor operation, meaning that high level waste is relieved of the long-lived isotopes and is reduced to small quantities of short-lived fission products. They can also turn uranium into a seemingly renewable resource, by producing more fissile material from the uranium-238 blanket than is consumed in the reactor.
Drawing on past experience of fast reactor technology and nuclear technology in general, it is foreseen that fast reactors will play an increasingly important role in nuclear power alongside the thorium cycle to ensure the most efficient use of resources.